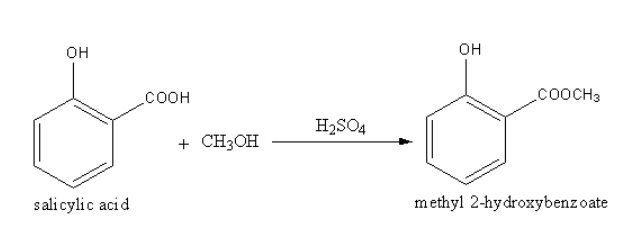
The carboxylic group -COOH converts into ester COC H 3 during the reaction of salicylic acid with methanol so the reaction is known as esterification. The influence of reaction temperature amount of catalyst duration of reaction and molar ratio of salicylic acid.

Write The Balanced Equation Of The Ester Prepared From Methyl Alcohol And Salicylic Acid Study Com
LB broth 25 ml was dispensed into each well of 12-well plates.
. In practice I decided to perform the reaction in a single flask and use concentrated sulfuric acid to. Fundamentally the reaction between methanol and salicylic acid in the presence of concentrated sulfuric acid and heat constitute an esterification reaction. Methanoldimethyl carbonate has been studied over sulphated zirconia.
Ht heat OH он 40. The influence of reaction temperature amount of catalyst duration of reaction and molar ratio of salicylic acid. The hydrolysis reaction that occurs will form methanol water and the sodium salt of salicylic acid.
Hence methanol appears to be a better methylating agent compared to dimethyl carbonate in terms of yield as well as atom economy. The second step is a standard Fisher esterification. The product methyl 2 hydroxyl benzoate is known as oil of wintergreen.
The mechanism of the reaction is the replacement of the OH hydroxyl group of the carboxylic. Up to 24 cash back Salicylic acid and methanol smell In this experiment you will do a series of chemical reactions designed to characterize alcohols aldehydes and ketones. Stock solutions 100 mM of salicylic acid and its derivatives acetyl salicylic acid salicylamide methyl salicylate and benzoic acid were prepared in methanol.
It will be stoppered or corked and then heated in a water bath at 60 C for 2 hours to complete the reaction. Science Chemistry QA Library Salicylic acid is added into a test tube followed by methanol and sulfuric acid as the catalyst. With your instructors assistance place your salicylic acid sample in a flask and add 15 mL of the H 2 SO 4 C 2 H 5 OH mixture.
The reaction of carboxylic acid and alcohol leads to the formation of esters in a process called esterification. When salicylic acid reacts with methanol Methyl salicylate commonly known as Oil of Wintergreen is formed. Methanoldimethyl carbonate has been studied over sulphated zirconia.
Name of ester product. Reaction of methanol and salicylic acid Odor. Methanol is also known as methyl alcohol and wood alcohol.
The type of reaction may be known as condensation reaction because the small molecule of H2O is eliminated from the reactants while the remaining bits of the reactants condense. It is heated for 10-15 minutes and poured into a beaker with crushed ice. Wintergreen oil is methyl salycilate which is the methyl ester of salicylic acid.
The esterification reaction is a form of condensation reaction because there is the formation of water molecules. Preparation of Soap by Saponification of a Triacylglycerol Saponification reaction and recovery of soap product and synthesis procedure and observations. Give one use of this ester in everyday life.
When an acid containing the COOH group reacts with an alcohol a compound containing an OH group formed an ester. Filter-sterilized stock was added to a 12-well plate to a final concentration of 01 05 or 1 mM. The chemical formula of Methyl salicylate is C6H4 OHCOOCH3 It can also be written.
Click to see full answer. Select the compound which will not show esterification reaction with H. Methanol on heating with salicylic acid and a few drops of conc.
Place 065 g of salicylic acid 20 mL methanol and a spin vane in a 5-mL conical vial. When salicylic acid is reacted with methanol in an acidic medium preferably sulphuric acid in the presence of heat a dehydration reaction occurs with the loss of water OH-ion is lost from the carboxylic acid functional group present in the salicylic acid molecule and H ion is lost from the deprotonation of the methanol molecule resulting in the formation of methyl salicylate an. The mechanism of action is dependent on the acidic conditions and heat.
In the reaction of salicylic acid and methanol to form methyl salicylate where does the oxygen in methyl salicylate indicated in the structure below come from. Be sure to label it with the name of your group. Salicylic acid methanol C8H10O4 CID 70258741 - structure chemical names physical and chemical properties classification patents literature biological.
Therefore this esterification reaction was first used in 1899 by the Bayer company to replace the hydroxyl group in salicylic acid with. Give an equation for the reaction of salicylic acid 2-hydroxybenzoic acid and methanol in the presence of heat and an acid catalyst H2SO4. This reaction will connect the salicylic acid to the methanol with concentrated sulfuric acid as the catalyst.
Reaction chemical equation with structures. Graham Brittain Page 9 of 10 10172014 Discussion. Complete the reaction table above in order to.
You will be able to determine if a reaction has occurred by several. To form the ester you need to combine equimolar amounts of salicylic acid and methanol with a catalytic amount of acid and apply heat. A Esterification of Acetic Acid with 1-pentanol.
Later in the work-up the salt is acidified with sulfuric acid to convert the organic salt into the protonated carboxylic acid. Methyl salicylate oil of wintergreen is an organic ester. This is the esterification of Oil of wintergreen.
Place the conical vial in an aluminum block on a stirring hotplate and while stirring slowly and in small portions add 075 mL of concentrated sulfuric acid to the salicylic acidmethanol solution. Oil of wintergreen is a naturally produced organic ester. Esterification may be intermolecular or intramolecular.
Acid on reaction with alcohol form ester this reaction is called esterification. Write the complete reaction equation and describe the odor of the reactants salicylic acid and methanol and the products oil of. Write the mechanism for the Fischer esterification reaction of salicylic acid and methanol to form methyl salicylate 1C.
Stir the mixture until the salicylic acid dissolves. The reaction of salicylic acid C6H4 OHCO2H and methanol CH3OH forms this ester. YOU MUST DRAW THE CHEMICAL STRUCTURES TO RECEIVE CREDIT Equation.
When salicylic acid combines with methanol it becomes the ester known as methyl salicylate or oil of wintergreen. The reaction of salicylic acid and methanol is a condensation reaction because there is the formation of methyl salicylate and water. Salts of organic compounds usually are soluble in water or will dissolve in water with a bit of heating.
Methyl Salicylate Synthesis Miles Dai
Methanol On Heating With Salicylic Acid And A Few Drops Class 11 Chemistry Cbse
0 Comments